News & Events
Danilo D'Angelo receives poster prize at the International Max Planck Research School Symposium 2025

Danilo D’Angelo, a third-year Ph.D. student, was awarded a poster prize at the International Max Planck Research School Symposium 2025 "Molecules in Motion", recognizing his contributions to advancing targeted cancer research. His work centers on the biological characterization and crystallization of Akt, which is crucial in supporting the structure-based design of isoform-selective covalent allosteric inhibitors (CAAIs) that selectively target the Akt isoforms. Through detailed structural studies and functional assays, his research provides essential insights into the distinct biological roles of these isoforms in cancer progression and cellular metabolism. By facilitating a deeper understanding of Akt isoform behavior and enabling the design of selective inhibitors that engage a unique allosteric pocket, his efforts aim to develop more precise and effective cancer therapies with fewer off-target effects.
Alexandros Pervanidis Receives Poster Award at the 18th Day of Chemistry

Alexandros Pervanidis, a second-year Ph.D. student, was awarded a poster prize for his innovative research at the 18th Annual Day of Chemistry in Dortmund, Germany. His work focuses on the structure-based design of isoform-selective covalent allosteric inhibitors (CAAIs) targeting Akt2 and Akt3 to further investigate their implication in cancer progression and metabolism. By elucidating the distinct roles of these isoforms and designing inhibitors that selectively bind to a unique allosteric pocket, this work aims to improve cancer treatments. His research on the design and synthesis of new probes offers the potential for more targeted therapies with fewer side effects than conventional inhibitors while advancing our understanding of Akt isoform functions.
New Publication: Targeting Drug-Resistant GIST with Covalent Inhibitors
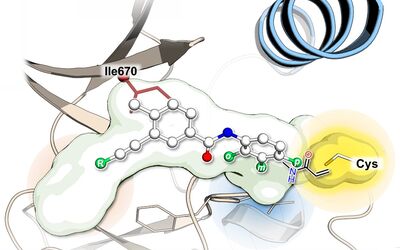
We are excited to announce our latest research publication in the Journal of Medicinal Chemistry! We developed novel covalent inhibitors targeting mutant versions of KIT and PDGFRA.
Why does this matter?
Gastrointestinal stromal tumors (GIST), the most common mesenchymal tumors of the digestive tract, are driven by activating mutations in the KIT and PDGFRA genes. While tyrosine kinase inhibitors (TKIs) have revolutionized treatment, drug resistance, and side effects remain major challenges. In this work, we developed covalent TKIs targeting KIT and PDGFRA drug-resistance mutations. Using a combination of structure-based drug design, organic synthesis, and biological evaluation, we identified promising inhibitors that effectively target mutant versions of these driver oncogenes. Most notably, we report the first crystal structure of PDGFRA bound to a covalent inhibitor, providing key insights into how these small molecules interact with their targets. Our findings highlight the promise of covalent inhibitors to overcome drug resistance and advance safer, more effective therapies for advanced GIST.
Read the full paper here: https://doi.org/10.1021/acs.jmedchem.4c02472
Vielversprechende Substanz gegen seltene Krebserkrankung auslizenziert
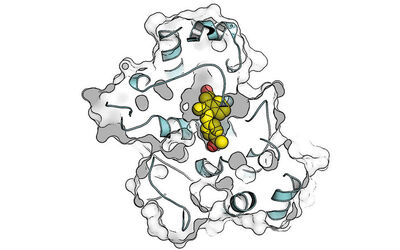
Rund 1.200 Menschen erhalten in Deutschland jährlich die Diagnose „Gastrointestinaler Stromatumor“, kurz GIST – eine seltene Krebsart, bei der die Tumoren in den Wänden der Verdauungsorgane entstehen und die schnell Resistenzen gegen gängige Präzisionsmedikamente entwickelt. Wissenschaftler*innen der TU Dortmund, des Westdeutschen Tumorzentrums am Universitätsklinikum Essen und des Dortmunder Max-Planck-Instituts für molekulare Physiologie haben einen vielversprechenden Wirkstoff gegen GIST identifiziert, zum Patent angemeldet und an ein US-amerikanisches Pharmaunternehmen lizenziert, das ihn nun bis zur Marktreife weiterentwickeln will – ein wichtiger Schritt auf dem Weg von der Grundlagenforschung zur klinischen Anwendung.
Nationales Netzwerk für die Entwicklung von Krebsmedikamenten gestartet
Die Deutsche Krebshilfe fördert das nationale Forschungsnetzwerk „TACTIC“ mit 11,8 Millionen Euro über fünf Jahre. Das Aufgabenspektrum der insgesamt 24 beteiligten Wissenschaftler*innen reicht dabei von der Entdeckung und Weiterentwicklung neuer Wirkstoffe bis hin zu präklinischen Studien. Die Arbeitsgruppe um Prof. Daniel Rauh von der Fakultät für Chemie und Chemische Biologie der TU Dortmund ist an TACTIC beteiligt und erhält für ihre Forschung 1,4 Millionen Euro von der Gesamtfördersumme.
Mehr dazu lesen Sie hier.
